Academics
| Degree | University/Institution |
|---|---|
| Ph. D., FNASc. | Dept. of Microbiology and Cell Biology, Indian Institute of Science, Bangalore, India. |
Work Experience
| Position | University/Organisation | Period |
|---|---|---|
| Scientist-G | Institute of Life Sciences, Bhubaneswar, Odisha. | Since Feb. 2025 |
| Scientist-F | Institute of Life Sciences, Bhubaneswar, Odisha. | Jan. 2021-Jan 2025 |
| Scientist-E | Institute of Life Sciences, Bhubaneswar, Odisha. | Jan. 2016-Dec. 2020 |
| Scientist-D | Institute of Life Sciences, Bhubaneswar, Odisha. | June 2011- Dec. 2015 |
| Research Scientist | Department of Biochemistry and Molecular Biology, University of Texas Medical Branch, Galveston, Texas | 2009-2011 |
| Post-Doctoral Fellow | Sealy center for Molecular Sciences, UTMB, Galveston, Texas | 2003-2009 |
Awards & Recognition
| Details |
|---|
|
Research
| Details |
|---|
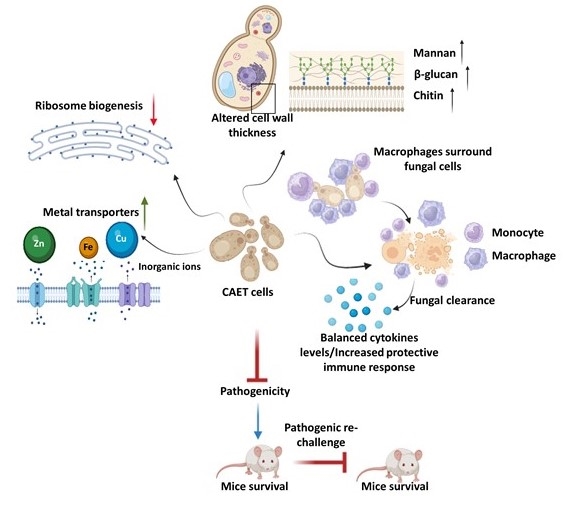
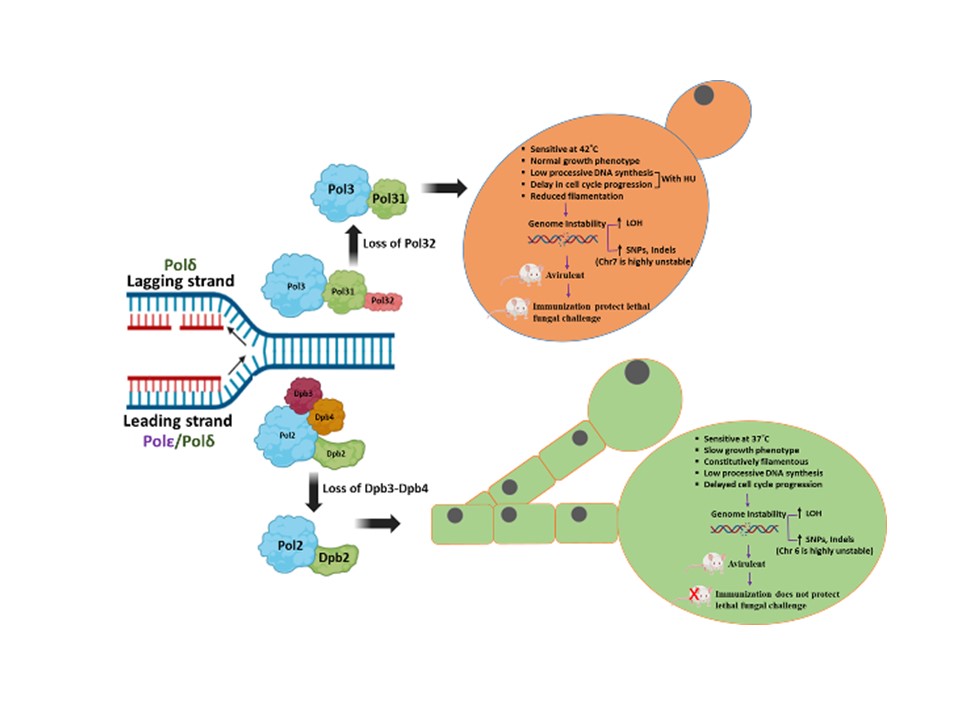
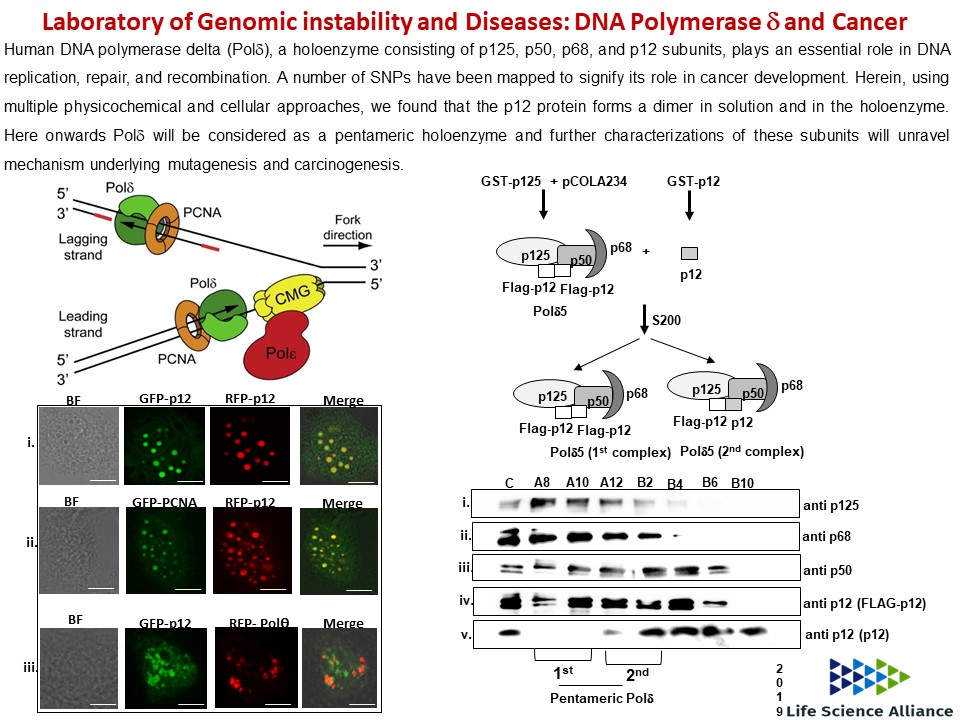
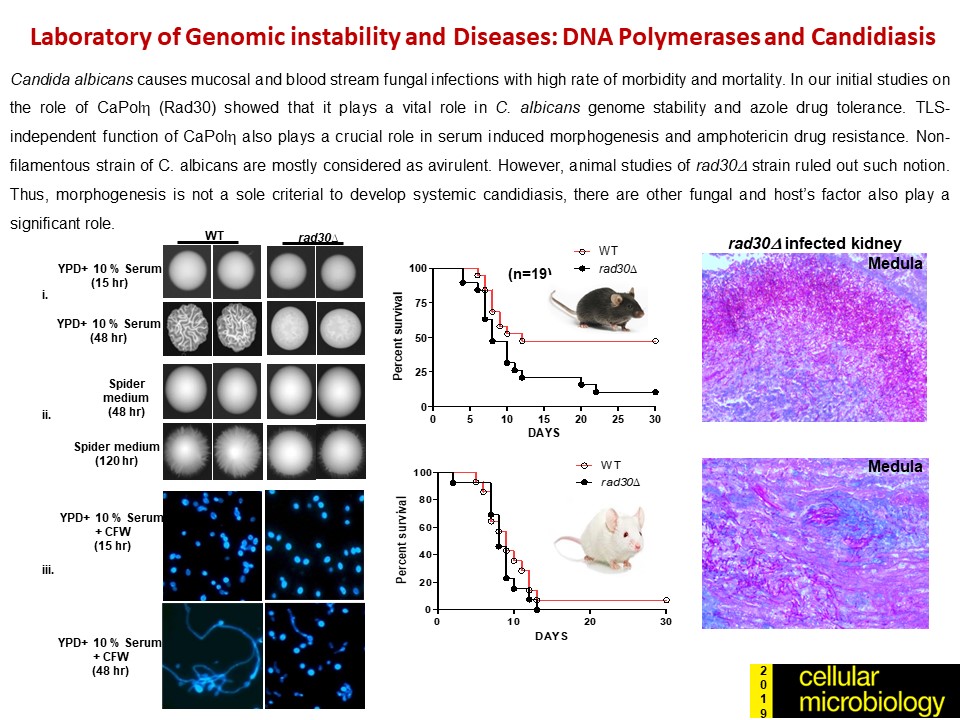
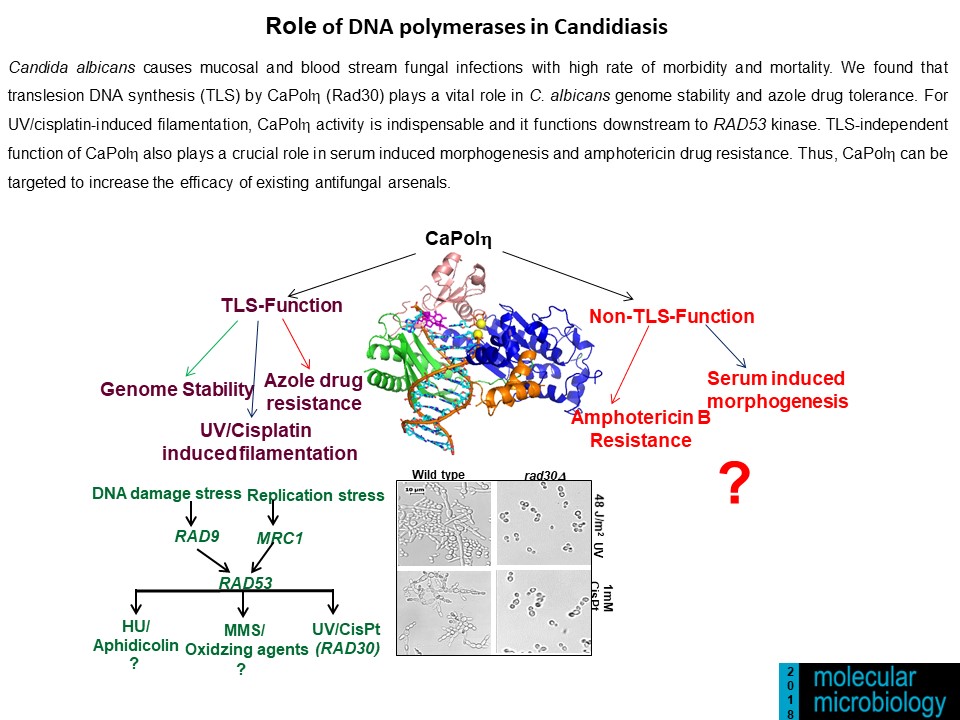
Genomic Instability and Diseases Research Summary: Replication of DNA strands during each cell division is a prerequisite and critical for proper functioning of any genome. It necessitates faithful duplication of the genetic content and ensures genome stability by preventing faulty DNA synthesis that could render spontaneous mutation, genome rearrangement, and DNA breaks. DNA polymerases (Pol) are the enzymes required for DNA synthesis virtually in all the DNA transaction pathways, and any abnormalities in Pol function has been shown to be associated with cell death, tumorigenesis, resistance to chemotherapeutics, and development of an array of complex diseases in humans. In microorganisms, genome instability is also linked to virulence and multidrug resistance. We focus on the mechanism of eukaryotic DNA replication and use both yeasts and human cells as our model systems. Genetic and biochemical studies in S. cerevisiae have indicated that the highly accurate and processive genomic DNA replication is carried out by the coordinated action of Polα-primase, Polδ and Polε (Biochemical Society Trans. 2020 and Current Genetics 2020). PCNA (the eukaryotic clamp loader) is an essential homotrimeric ring protein plays a critical role in orchestrating the assembly of replisome complex, recruiting DNA polymerases, and factors involved in DNA damage response, chromatin assembly and remodeling, and cell cycle control. Each of the PCNA-binding partners harbors a dedicated region called PCNA-interacting protein (PIP) motif. PIPs in all three subunits contribute to processive DNA synthesis by Polδ, and mutational inactivation of all three PIP motifs abrogates its ability to synthesize DNA with PCNA (PNAS, 2011). In human, unlike in yeast, Polδ is a pentameric holoenzyme consisting of p125, p50, p68, and dimeric p12 subunits. An amino-terminal dimerization and a carboxyl terminal PIP motifs in p12 have been mapped, and dimerization of p12 is critical for its interaction with PCNA (LSA, 2019). Although PCNA is a structurally conserved protein, complementation analyses suggest it to be highly species specific (BMC-Micro. 2015 and FEBS letters 2021). By using structural and mutational analyses, we find that the IDCL and J loop structures of PCNA are unique and responsible for cross-species incompatibility, therefore these regions may be targeted to develop species-specific inhibitors (JBC(a)-2021). DNA replication does not proceed smoothly, as it encounters both DNA lesions and non-B form of DNA structures that must be by-passed to prevent collapsing of replication fork. Trans-lesion DNA synthesis (TLS) polymerases are highly sophisticated enzymes can bypass certain lesions in DNA. DNA polymerase eta (Polη) is one such enzymes required for efficient bypass of UV- induced cyclobutane pyrimidine dimers in the genome. Additionally, Polη also possess non-TLS activities such as serum induced morphogenesis, amphotericin B drug resistance and virulence in pathogenic yeast Candida albicans (Molecular Micro.- 2018, Cellular Micro. 2019, and Current Genetics 2019). Further, we identify three distinct group of Polηs from species across kingdom based on their modes of PCNA interaction (JBC (b)-2021). Candida albicans survives as a commensal fungus in the gastrointestinal tract, and its excessive growth causing infections in immune-suppressed individuals is widely accepted. However, our recent data suggests a mutualism between the host and C. albicans. Dietary C. albicans does not cause any noticeable infections, rather it colonizes in the gut to modulate microbiome dynamics which in turn negates the high-fat diet induced uncontrolled body weight gain, metabolic hormonal imbalances, and inflammatory responses. Interestingly, adding C. albicans to a non-obesogenic diet stimulates the appetite regulated hormones and helped the mice gain healthy body weight (biorxiv-2022/M.Spectrum 2022). In its pathogenic state, C. albicans causes fatal systemic infections and are associated with a higher rate of morbidity and mortality, and represent major healthcare problems. Currently, chemotherapy is the sole available option for combating fungal diseases and no vaccine has been developed yet for human use (FCIM-2022). We have generated several genetically engineered knockout strains of C. albicans and also identified few of those strains to be attenuated (Gut microbes-2023). Two of such isolates [CNA94-Indian Patent Application No.: 202231013971 (JBC-2024-Cover page) and CNA25- Indian Patent Application No.: 202231043203 (EMBO Molecular Medicine)] have also been patented to explore further as a whole cell vaccine against fungal and other cross-species infections. In addition a detailed characterisations of EDTA-treated C. albicans cells both in vitro and pre-clinical models led to discovery of a potential live whole-cell vaccine against fungal infections (Indian Patent Application No.: 202331056818, eLife-2024). |
Publications
| Details |
|---|
Genomic Instability and Diseases
Peer reviewed publications:
Pre-prints
Book chapter
|
Group
| Details | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Laboratory of Genomic Instability and DiseasesPh.D. students:
Lab. Technician:
Our Graduates:
Our Alumni Dr. Abinash Dutta, Ph.D. (DBT Project RA- from 2021-Sept. 2024) Dr. Shweta Thakur, Ph.D. (NPDF/DBT-RA- from April 2018- March 2024) Dr. Manish Singh, Ph.D. (DBT Project RA- 2021 (April-Nov.) Dr. Doureradjou Peroumal, Ph. D. (CSIR/DBT-RA- 2016-March 2021) Dr. Shreenath Nayak, Ph.D. (DBT-RA; from the year 2013-2016) Dr. Jawed Alam, Ph.D. (CSIR-RA-2015-16) Amrita Dalei (Lab. Technician – 2013-2018) Short-term Fellows (2-6 months):Subhalaxmi Moharana ((M. Sc. Dissertation -2024- Central University, Gujarat) Aradhana Mishra (M. Sc. Dissertation -2023- Trident. BBSR) Aishi Satpathy (M. Sc. Dissertation-2019, Central University of Tamil Nadu) Rituparna Moharana (M. Sc. Dissertation-2019, S & O University) Manoj Kumar (IAS Fellow – 2019, Anna University Regional Campus Coimbatore) Mousumi Sajjan (Summer project-2018,OUAT, BBSR) Riya Gupta (IAS Fellow 2017,M. Tech. Biotechnology, Meerut Institute of Engineering and Technology ) Ravi Kumar (IAS Fellow 2016, NIT, Durgapur) M. Suresh (IAS Fellow 2015) Best Poster Award (YIM-2023-IISER, Mohali)
|
Grants
| Details |
|---|
Genomic Instability and DiseasesOngoing sponsored Research Projects:
Completed sponsored Research Projects:
|
Contacts
| Address | Fax | Office | |
|---|---|---|---|
| narottam_acharya@ils.res.in | Nalco Square, Bhubaneswar-751023, India | 0091 674 2300728 | 0091 674 2304278 |
Highlights
| Details |
|---|
Lab. in News https://vigyanprasar.gov.in/isw/Study-paves-way-for-a-new-approach-to-fight-infections.html
eLIFE-2024
mBio-2024
|
























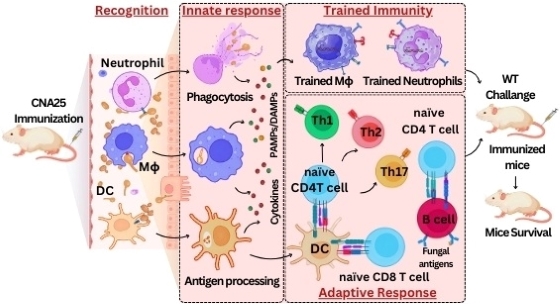 EMBO MOLECULAR MEDICENE- 2024
EMBO MOLECULAR MEDICENE- 2024